Simulating blood vessel scaffolds
Three-dimensional simulations of drug-releasing blood vessels can predict the release pattern of drugs into the bloodstream
Published online 30 March 2015
Angioplasty are commonly used as a non-surgical intervention to treat several heart diseases.
© BSIP SA / Alamy
Computer simulations of how drug-releasing stents interact with flowing blood immediately following implantation may improve design of such devices, as well as help predict the outcomes of patients in whom they are implanted, according to a new study by researchers at Emory University1.
Stents are usually made of metallic scaffolds, and are commonly implanted into heart disease patients, by a procedure called angioplasty, in order to widen narrowed arteries and alleviate symptoms such as chest pain.
In recent years there have been a number of important innovations in stent design, including a reduction in the size of the devices, and the development of stents made of absorbent materials that can be coated with drugs. Nevertheless, implantation still carries risks, as it often causes inflammation. Stents also affect the function of blood vessels function by altering their diameter, and blood clots can sometimes form inside them following transplantation.
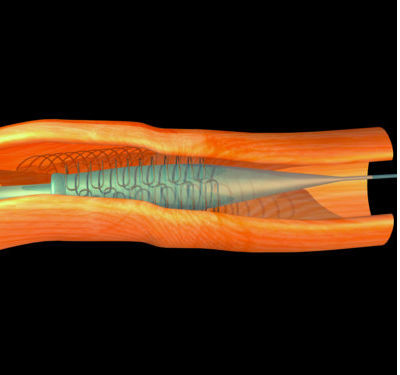
Habib Samady and his colleagues used computational fluid dynamics to generate three-dimensional simulations of how drug-releasing bioresorbable scaffolds interact with vessels and affect blood flow. These devices are designed to release drugs slowly and then dissolve or be absorbed by the tissues surrounding them.
The researchers simulated the effects of a stent positioned in various locations of straight and curved blood vessels, focusing on the amount of shear stress the device places on the walls of the vessels, and how this affects blood flow.
According to their simulations, the amount of shear stress is highest at the endoluminal strut surface, where the stent comes into contact with the inner lining of the vessels, but very low at the areas where blood flows in and out of the device.
At 33% bioresorption of the stent, the picture began to change. The model showed that the amount of stress at the endoluminal surface — where the stent comes into contact with the side of the vessel — is reduced by about one third, but increases by more than 100% at the in-flow and out-flow regions, leading to a more uniform distribution of stress across the device at this stage.
The model also revealed that these effects were more pronounced at the inner than the outer curvatures of curved vessels.
Reference
- Gogas, B. D., Yang, B., Passerini, T., Veneziani, A., Piccinelli, M., et al. Computational fluid dynamics applied to virtually deployed drug-eluting coronary bioresorbable scaffolds: clinical translations derived from a proof-of-concept. Glob. Cardiol. Sci. Pract. 2014, 56 (2014). | article
DOI: 10.1038/qsh.2015.54

